The researchers from St. Petersburg, Russia have used the antiviral properties of NSC 631570 in their works with viral hepatitis C (HVC). They reveal NSC 631570, at optimal dosage, has brought about the elimination of the virus from the blood in 40 (80%) of 56 patients (165).
In their further study these researchers compared the effects of NSC 631570 at various dosages with the recombinant human interferon-alpha-2b (IFN) in 75 HVC patients. The best results were achieved when NSC 631570 was administered at a single dose of 1 mg (203).
Pilot studies evaluating the effect of NSC 631570 on HIV virus and related diseases were also performed (12). The authors noted, for example, improved immune values after the therapy (103).
The antiviral properties of NSC 631570 were confirmed in the in vivo experiments (42, 51, 52, 88, 90).
THE INHIBITION OF THE TUMORAL ANGIOGENESIS
NSC 631570 inhibits the formation of the new blood vessels supplying a tumor. Due to these antiangiogenic properties NSC 631570 administered before surgery brings about better demarcation of the tumor from surrounding tissue and the tumor encapsulation. This alleviates the surgical removal of tumors what has been confirmed in breast cancer studies (68-73, 114). It is recommended to reduce the tumor burden 7-10 days after the start of the therapy with NSC 631570.
In tests in vitro, NSC 631570 inhibited dose-dependent the proliferation of human endothelial cells without exerting cytotoxic effect. The angiogenesis inhibition was observed on the capillary formation model. The tumor angiogenesis is the formation of new blood vessels supplying a growing tumor with nutrients. Angiogenesis is of critical importance for the tumor growth (136).
Inhibition of the capillary sprouting by NSC 631570 in the endothelial spheroids model

Pancreatic adenocarcinoma, preoperative treatment with Ukrain, total 100 mg, 10 days prior to surgery (pancreatico-duodenectomy). Formation of a capsule (A) around the tumour. Tumour cells do not infiltrate the capsule. Massive round cell infiltration (B) of the tumour–capsule border area. H-e, x100.

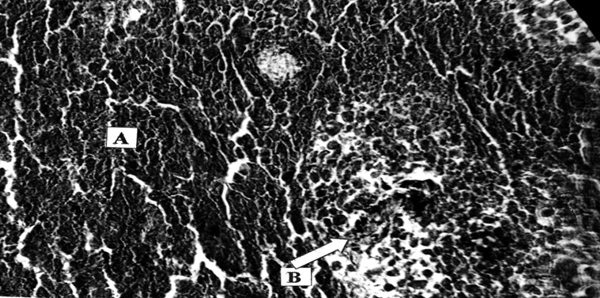
Pancreatic adenocarcinoma, preoperative treatment with Ukrain, total 100 mg, 10 days prior to surgery. Necrosis of tumour tissue (A), inhibition of new vessels formation (defect in capillary wall, defect in endothelial covering, B). H-e, x200.
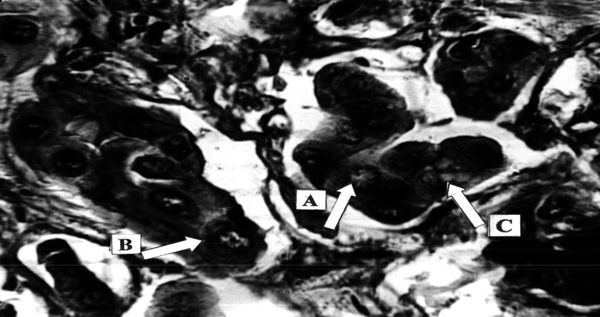
Pancreatic adenocarcinoma, preoperative treatment with Ukrain, total 100 mg, 10 days prior to surgery. Nuclear changes: Chromatin dispersion (A) and fragmentation (B), hydropic cytoplasm degeneration (C). “Iron” hematoxylin – van Gieson, x500.
THE IN VITRO STUDIES
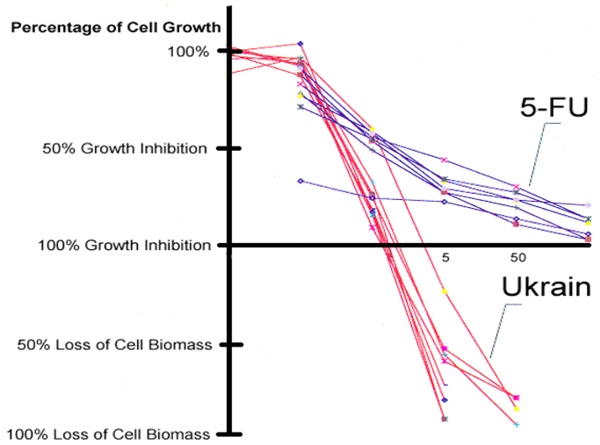
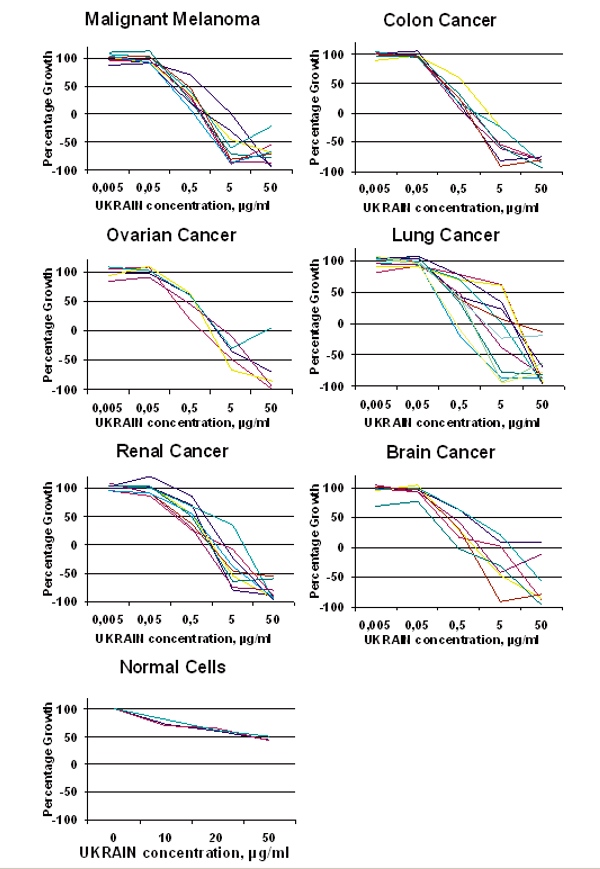
Clinical efficacy of NSC 631570 is not coincidental or even ‘spontaneous remission’ but rather a consequence of its mechanisms of action confirmed in various in vitro and in vivo studies. NSC 631570 has been tested on more than 100 cancer cell lines so far.Among others, NSC 631570 was tested at the National Cancer Institute (Bethesda, Maryland, USA) on 60 cell lines representing eight important human malignant tumors: brain tumors, ovarian, small cell and non-small lung cancer, colon cancer, kidney cancer, leukaemia and malignant melanoma. NSC 631570 exerted toxic effects against all these cell lines (40, 190). Compared to 5-fluorouracil (5-FU) and gemcitabine, two standard cytotoxic agents in the treatment of digestive tract tumors, NSC 631570 achieved better results and not only inhibited the cell growth but reduced the cell mass, also.
The cytotoxic effect of NSC 631570 on the cancer cell lines M-HeLa and Hep-2/0-6-5 war more pronounced than the effect of a synthetic agent Oliphen (61). NSC 631570 inhibited the growth of four Ewing’s sarcoma cell lines dose- and time-dependent. In this experiment the effect of NSC 631570 was more pronounced than this of thiotepa (243, discussed in 244). In the tests on Ehrlich’s carcinoma cells and lympholeucemia P-388, the researchers revealed that the sensitivity of the cancer cells to NSC 631570 depends highly on the cell cycle phase, the first sensitivity peak being at the end of G1 phase and the second - in G2 phase (148). The effects of glucose, succinat, pH value and increased temperature on the efficacy of NSC 631570 against cancer cells were investigated in vitro. Glucose reduced the cytotoxic effect of NSC 631570, but succinat intensified it. The most pronounced effect was at pH 7.3-8.0. The temperature up to 41.5 °C did not impact the effect of NSC 631570 (117).
Results of the Ukrain (NSC 631570) study at the National Cancer Institute, Bethesda, Maryland, USA. The effects of NSC 631570 on the 60 various human cancer cell lines
The Selective Effect of NSC 631570
In comparative studies NSC 631570 was tested on 18 malignant and 12 non-malignant cell lines at identical conditions (36, 38, 63, 143, 147, 149, 181, 184, 190, 245, 255). These experiments eliminated all doubts on the selective effect of NSC 631570. Numerous studies have confirmed NSC 631570 to be the first and only anticancer agent being toxic against cancer but not normal cells. This explains also its good tolerability in clinical use.
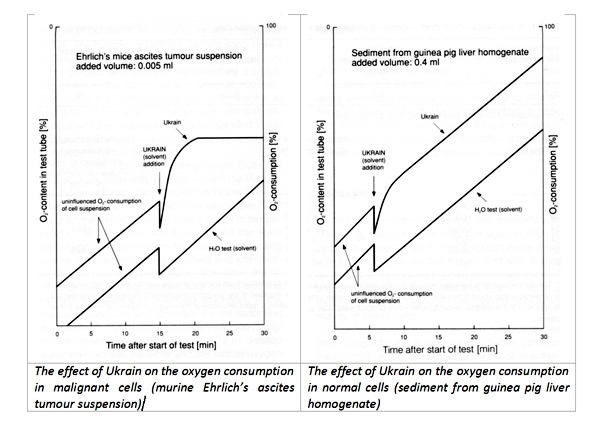
First indications on the selective effect of NSC 631570 on the cancer cells provided an early study in 1976 of the Bundesstaatlichen Anstalt für Experimentell-Pharmakologische und Balneologische Untersuchungen (Vienna, Austria). This study revealed different oxygen consumption by normal liver cells and Ehrlich’s tumor ascitic cells after the incubation with NSC 631570 was revealed (38).
About at the same time, the researchers from the Vienna University of Agriculture compared the inhibiting effect of NSC 631570 on the proliferation of malignant and normal cells. For 50% growth inhibition of normal endothelial cells, the NSC 631570 concentration had to be tenfold higher than for the same growth inhibition of human osteosarcoma cell line (36).
The different influences of Ukrain on oxygen consumption in healthy cells and cancer cells
Die Aufnahme von Ukrain in Melanom-Zellen, verglichen mit normalen Zellen (in vitro)
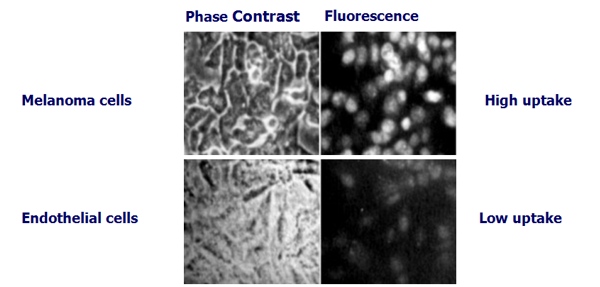
Hohenwarter O. et al. Selective inhibition of in vitro cell growth by the anti-tumour drug Ukrain, Drugs under Experimental Research, 1992 (36).
This selective effect of NSC 631570 on cancer cells has been confirmed in numerous studies at the renowned universities and research facilities.
In the study from European Organisation for Research and Treatment of Cancer (EORTC), human tumor xenografts (HTX) were implanted into nude mice. These tumor cells were later incubated with NSC 631570 at various concentrations. Following HTX were used: colon cancer CXF 1103/11, stomach cancer GXF 217/17, lung cancer LXFL 529/14, breast cancer MAXF 401/13, melanoma MEXF 276/10, and ovarian cancer OVXF 899/9. NSC 631570 was active against OVXF 899/9 at 10 µg/ml and in all colonies tested at 100 µg/ml with the T/C-ratio (‘test to control’) 1/135 in OVXF, 8/109 in CXF, 10/98 in GXF, 15/187 in LXFL, 34/133 in MAXF, and 10/122 in MEXF (64, 189).
In 1998, at the 89th Annual Meeting of the American Association for Cancer Research in New Orleans, USA, researchers from the University of Pretoria, South Africa presented their work on the selective effect of NSC 631570 on various cancer cell lines. The authors concluded ‘that Ukrain is selectively toxic to malignant cells by causing a metaphase block which is characterised by abnormal chromosomal distribution, and results in the formation of micronuclei and in apoptosis’ (139). In 2000 the same group discovered NSC 631570 to inhibit the tubulin polymerisation. In this paper they denied the selective mode of action of NSC 631570 on cancer cells (140).
Researchers from Eberhard-Karls-University Tubingen, Germany, investigated the effects of NSC 631570 on cell survival, alteration of the cell cycle and induction of apoptosis without and in combination with ionising radiation (IR) at a dose of 1-10 Gy. The tests were performed on the exponentially growing human tumor cells MDA-MB-231 (breast), PA-TU-8902 (pancreas), CCL-221 (colon cancer), U-138MG (glioblastoma), and human skin and lung fibroblasts HSF1, HSF2 and CCD32-LU. Without IR, NSC 631570 exerted a time- and dose-dependant cytotoxic effect, more pronounced against the cancer cells. Flow cytometry revealed NSC 631570 to modulate radiation toxicity against human cancer cell lines and to protect normal cells from radiation. The combination of NSC 631570 plus IR gave enhanced toxicity in CCL-221 and U-138MG cells with their accumulation in the G2/M phase of the cell cycle, but not in MDA-MB-231 and PA-TU-8902 cells. A radio protective effect was found in normal human skin and lung fibroblasts. The authors suggest a reasonable use of NSC 631570 in combined radiochemotherapy (184).
The cytotoxic effects of NSC 631570 were evaluated in two primary pancreatic cancer cell lines (PPTCC), fibroblasts derived from pancreatic ductal adenocarcinoma specimens (F-PDAC), and an immortalized epithelial ductal pancreatic cell line (HPNE). Cytotoxicity was assessed by the CellTiter 96 kit based on the cellular metabolism of the tetrazolium compound XTT, which is reduced by living cells to yield a soluble formazan product in the presence of the electron coupling agent phenazine methosulfate, while the modulation of NSC 631570 uptake in the medium was studied using the fluorescence of NSC 631570 with the AlphaDigiDoc software by UV light excitation. Cytotoxic effects of NSC 631570 in PPTCCs were significantly higher than those observed in F-PDAC and HPNE cells (20% vs. 80% alive cells). Furthermore, the ULA-DC test revealed that PPTCCs cells consumed more drug than F-PDAC and HPNE cells. These data demonstrated the selective effect of Ukrain in PPTCCs, which may be related to a different transport system or higher metabolism of the drug in PDAC, and warrant further investigations in order to support the possible role of Ukrain in PDAC treatment (265).
Induction of the Apoptosis in Cancer Cells
In the study on the erythroleucemia cells K-562, it was revealed NSC 631570 to bring about the bimodal death of cancer cells. At lower concentrations of NSC 631570, cancer cells die in as a consequence of apoptosis. At higher concentrations, the formation of microtubules is inhibited and polyploidy occurs (56, 62).
The researchers from the Rochester University, USA revealed NSC 631570 causes the accumulation of prostate cancer cells as well as epidermoid carcinoma cells in the G2/M phase, however, not of normal cells (149, 150).
In the tests on human cervix carcinoma cells HeLa, squamous carcinoma cells WHCO5, normal kidney cell line Graham 293, and transformed kidney cell line Vero from African green monkey the researchers of the University of Pretoria, South Africa revealed NSC 631570 ‘is selectively toxic to malignant cells by causing a metaphase block which is characterised by abnormal chromosomal distribution, and results in the formation of micronuclei and in apoptosis’ (139).
The scientists of the Eberhard Karl University (Tübingen, Germany) investigated the effect of NSC 631570 on the cell survival, the cell cycle modification and the apoptosis induction alone and combined with radiation (IR). They discovered NSC 631570 combined with IR increased the toxicity against the cell lines CCL-221 and U-138MG. The normal human skin and lung fibroblasts were protected from the damaging effects of IR (184).
Ukrain’s (NSC 631570) deadly action against cancer cells - Induction of apoptosis
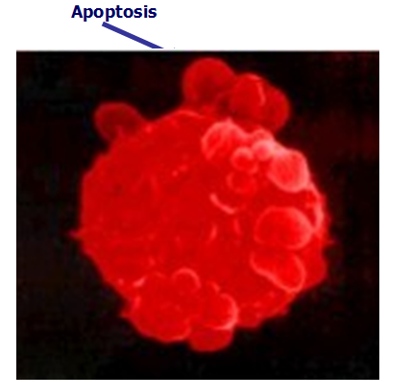
Estimating the cell proliferation according to the BrdU uptake in the cell lines AsPC1, BxPC3, MiaPaCa2, Jurkat, and THP-1 and the cell cycle phases by means of Giemsa staining, the authors established NSC 631570 at a dose of 10 µg/ml brought about a considerable accumulation of cancer cells in the G2/M phase after 24 h incubation. The apoptosis rate in the peripheral mononuclears was similar at the same incubation conditions. Moreover, the mitogene stimulated lymphocytes showed increased blastogen reaction (181).
The apoptosis induction by NSC 631570 has been also confirmed in the in vitro tests on the Chinese hamster ovarian cells. In this experiment the effects of NSC 631570 and etoposide were synergistic (167).
Inhibition of the Tubulin Polymerisation
In the experiments with cow brain tubulin, the scientists from the Pretoria Universioty (South Africa) discovered NSC 631570 to inhibit the tubulin polymerisation (146).
Later on, the researchers from the University of Ulm revealed in the tests with pancreas cancer lines AsPC1, BxPC3, Capan1, MiaPaCa2, and Panc1 NSC 631570 brings about a dose dependant cell cycle arrest in the G2/M phase. Moreover, NSC 631570 stabilizes the tubulin monomers in cancer cells and consequently inhibits the formation of microtubules (143).
Activation of Mitochondrial Caspases
Caspases, or cysteine-aspartic proteases are a family of enzymes, which play essential roles in apoptosis (programmed cell death) hence in cancer therapy.
In the tests on the Jurkat lymphoma model, NSC 631570 has been proven to be a strong apoptosis inductor. Profound research showed NSC 631570 brought about the depolarisation of mitochondrial membranes and consequently the activation of caspases (246).
There are two types of apoptotic caspases: initiator (apical) caspases and effector (executioner) caspases. Initiator caspases CASP8 and CASP10 induce the death factor dependant or extrinsic apoptosis pathway, whereas effector caspase CASP9 mediates the intrinsic or mitochondrial apoptosis. In the tests at the Nacional de Cancerologia, Mexico City, Mexico NSC 631570 revealed that Ukrain induced apoptosis in a panel of cancer cell lines (cervical cancer HeLa, HeKB, HeKS32, HeBcl3, HeNFR and HeIKK, human colon cancer SW480, human renal carcinoma HEK293, human osteosarcoma MG-63) by activating the intrinsic cell death pathway. Interestingly, non-transformed fibroblasts hTERT were insensitive to the drug (255).
The Effect on Cyclins and Cyclin-Dependant Kinases
Cyclins are a family of proteins which control the progression of cells through the cell cycle by activating cyclin-dependent kinase (Cdk) enzymes. A cyclin forms a complex with Cdk. Complex formation results in activation of the Cdk active site. When concentrations in the cell are low, cyclins dissociate from Cdk, thus inhibiting enzymatic activity; this probably occurs due to a protein chain of the Cdk blocking the active site upon cyclin dissociation. There are several different cyclins which are active in different parts of the cell cycle and which cause the Cdk to phosphorylate different substrates. Increasing concentration of cyclin A triggers the cell into the G2 phase, whereas cyclin B is essential for the mitosis initiation.
The researchers from the Rochester University explored the effect of NSC 631570 on cyclins and Cdk in the epidermoid cancer cell lines ME180 and A431 as well as in the prostate carcinoma line LNCaP. They found changes in the concentrations of mitotic cyclin A and B1 as well as Cdk1 and Cdk2. Increased expression of Cdk inhibitor p27 has been also observed in both cancer cell lines. This can promote accumulation of the cells in the G2/M phase (147, 149).
The Effect on the Expression of hENT1 and dCK
The interactions between NSC 631570 and the molecular determinant expressions involved in the metabolism of gemcitabine, such as hENT1 and dCK were evaluated in in vitro studies on pancreatic ductal adenocarcinoma cells (PDAC). Two ATCC cell lines (PL45 and MiaPaCa-2) and 2 Primary Cell Cultures obtained from PDAC patients underwent surgical resections (PPTCC78 and PPTCC109) were used. Cells were treated with Ukrain at IC50 concentration levels for 48h. All the amplifications were carried out with normalization of gene expression against the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) housekeeping control gene, and the quantitation of gene expression was performed. NSC 631570 positively modulated the expression of hENT1 mRNA in all PDAC cell cultures treated with IC50 (p<0.001). The analysis revealed a mean increase of 2.8 fold (p=0.001) compared to untreated control cells. In PL45 and MiaPaCa-2 cells NSC 631570 positively affects mRNA expression of dCK gene as well (264).
Based on the previous clinical data the NSC 631570-gemcitabine combination appears a promising regimen and the results of this study provided the experimental basis for the further clinical testing of the NSC 631570-gemcitabine schedule in PDAC patients (Funel et al, ‘Molecular mechanisms underlying the synergistic interaction of the novel anticancer drug Ukrain with gemcitabine in preclinical models of pancreatic cancer’, 44th Annual Pancreas Club Meeting, New Orleans, USA 2010).
The Effect on the DNA and Protein Synthesis
The effect of NSC 631570 on the DNA and protein synthesis in cancer cells was studied in vitro on the cell lines HeLa, Yoshida, mice lymphoma, mice myeloma, human WiDr tumor, EB, EsB, YAC-1, und P815. NSC 631570 (1-100 µg/ml) has been proven to inhibit in dose-dependant way the DNA, RNA and protein synthesis in these cell lines. At the same test conditions, the inhibition in normal cells (human tonsil cells and guinea pig liver cells) was much lesser distinct (58, 63).
Among many possible mechanisms of action of NSC 631570, its effect on the endonucleases of the rat liver and on topoisomerases was studied (topoisomerases play an important part in the DNA metabolism). In these experiments NSC 631570 inhibited the metal-dependant endonucleases and the topoisomerase I as well (166).
The Effect on the Proteins Involved into the Remodelling of Extracellular Matrix
An Italian research team from the University of Milan used RT-PCR, Western blot analysis and SDS-zymography to evaluate the effect of NSC 631570 on the expression of genes and proteins involved into the remodelling of the extracellular matrix. This mechanism plays an important role in the tumor invasion of human glioblastoma cells. There was a significant dose-dependant decrease of the proliferation of glioblastoma cells and a trend to the down-regulation of secreted protein acidic cysteine rich (SPARC). This study provided theoretical reasons for the using NSC 631570 in glioblastoma. The authors concluded: NSC 631570 ‘may be a useful therapeutic tool for brain tumors’ (245). This has been proved in clinical use (see “Brain tumors”).
In their further work with glioblastoma cells the Italian researchers revealed NSC 631570 to increase the expression of the glial fibrillary acidic protein (GFAP). The connexion 43 expression was not modulated by NSC 631570. These results ‘support the possible potential of NSC 631570 for the therapy of brain tumors’ (250).
This Italian team investigated also whether NSC 631570 is able to modulate the in vitro expression of some proteins involved in tumor progression on three clear cell type renal carcinoma cell lines (ccRCC). CAKI‐1, CAKI‐2 and ACHN clear cell carcinoma (ccRCC) cells were treated with three doses of NSC 631570 (5, 10, and 20 μM) or left untreated, and cultured for 48 h in duplicate. SPARC protein levels were assayed in Western blot, MMP‐2 and MMP‐9 protein levels and activity were determined by SDS‐zymography in cell culture supernatants, and the distribution of the different cell cycle phases was calculated using FACS analysis. The results suggest that NSC 631570 modulates two major aspects involved in tumorigenesis of RCC cancer cells, that are extracellular matrix remodelling and cell proliferation. The tendency to MMP‐2 and MMP‐9 down‐regulation in UK‐treated cells suggests that UK may decrease RCC cell invasion. Moreover, SPARC protein down‐regulation in supernatants point to an inhibition elicited by UK also on extracellular matrix remodelling in the tumor microenvironment, possibly rendering the tumor microenvironment less permissive for tumor invasion. At the same time, SPARC intracellular protein levels up‐regulation in ccRCC cells suggest that NSC 631570 may be involved in the inhibition of cell proliferation by cell cycle inhibition, as shown by FACS analysis. (Pettinari et al, ‘Matrix metalloproteinases activity and SPARC expression are targeted by Ukrain administration in renal cell carcinoma’, presentation at the symposium ‘Targeting Cancer Invasion and Metastasis’, Miami, USA, 2010).
The effect of NSC 631570 on the modulation of some of the key markers of tumor progression in pancreatic carcinoma was investigated on three cell lines HPAF-II, PL45, and HPAC. The cell lines were treated with NSC 631570 (5, 10 and 20 µM) for 48 h, or left untreated. Secreted protein acidic and rich in cysteine (SPARC) mRNA levels were assessed by real-time PCR. Matrix metalloproteinases (MMP)-2 and -9 activities was analyzed by SDS zymography; SPARC protein levels in cell lysates and supernatants were determined by Western blot. Cell cycle was determined by flow cytometric analysis, and invasion by matrigel invasion assay. NSC 631570 down-regulated MMP-2 and MMP-9, suggesting that this agent may decrease pancreatic cancer cell invasion, as confirmed by the matrigel invasion assay. SPARC protein downregulation in supernatants points to an inhibition NSC 631570 of extracellular matrix remodelling in the tumor microenvironment. At the same time, SPARC mRNA and cellular protein level up-regulation suggests that NSC 631570 can affect cell proliferation by cell cycle inhibition, showing a cell cycle G2/M arrest in UK-treated cells (266).
The Effect on the Heat Shock Proteins
In the tests with human lymphoma cells the effect of NSC 631570 on the lines U-937 and U-937/hsp70 was determinated. These cell lines differ in the expression of the heat shock protein 70 (Hsp70). The line U-937/hsp70 turned out to be more resistant against the effect of NSC 631570. The authors attribute this to the protective effect of Hsp70 (200).
The Effect on the Electrokinetic Potential
A study examined how NSC 631570 affects the electrokinetic (EKP) potential of malignant and benign cells. The EKP of the Ehrlich’s carcinoma cells dropped after incubation with NSC 631570. The EKP decrease of normal cells was less pronounced (221).